Electroplating: Difference between revisions
No edit summary |
m (Text replace - "[[Category:Power,_energy_&_industry_application" to "[[Category:Power,_energy_&_industry_applications") |
||
| Line 8: | Line 8: | ||
[[Category:Business,_management_&_industry]] | [[Category:Business,_management_&_industry]] | ||
[[Category:Power,_energy_& | [[Category:Power,_energy_&_industry_applications]] | ||
[[Category:Electrochemical_devices_&_processes]] | [[Category:Electrochemical_devices_&_processes]] | ||
[[Category:Batteries]] | [[Category:Batteries]] | ||
Revision as of 13:36, 13 November 2013
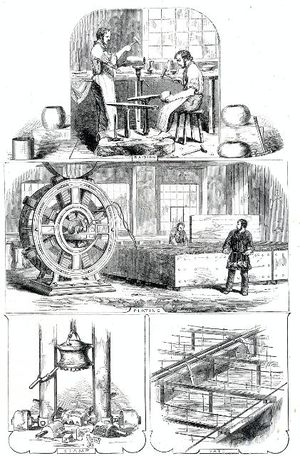
Electroplating is a process used to apply very thin coatings of metal onto objects of various kinds. It has many diverse purposes—it is used to make jewelry and flatware, but is also used to make car bumpers.
The Italian inventor Luigi V. Brugnatelli invented the art of electroplating in 1805. He connected a wire between a Voltaic pile (a battery) and a solution of dissolved gold. A wire connected to a metal object grounded the circuit, and as the current flowed the gold became attached to the surface of the metal object to make a smooth, shiny coating.
Electroplating became an important commercial process in the 1840s, when John Wright of Birmingham, England, discovered that gold or silver could be dissolved in potassium cyanide for use in electroplating. One of the first companies to use the new process was the English firm Elkington & Mason, which opened a silverware manufacturing company and made things such as eyeglass frames, pen nibs, and other small metal products that could be plated in large batches. Charles Christofle in France licensed the process from Elkington and achieved considerable commercial success plating hollowware and flatware.
For many years electroplating was used mainly to produce expensive-looking objects from inexpensive materials. In 19th century Russia, for example, thousands of religious icons, plated with gold or silver, were used in the nation’s churches. In later years, however, companies used electroplating to make objects that could not easily have been duplicated, even with expensive materials. A classic example is the automobile bumper. Large numbers of bumpers have been made since 1900 utilizing ordinary steel plated with chromium (or chrome), a metal with a high level of shininess that does not rust easily.